Principle
Use the difference in solubility of various components in plants in different solvents to select a suitable solvent to dissolve the target component from the plant raw material.
Common solvents
Water: safe and cheap, can extract water-soluble components such as sugars and proteins, but the extract is easy to deteriorate, and subsequent separation and purification are cumbersome.
Hydrophilic organic solvents: such as ethanol and methanol, have good solubility in plant components, high extraction efficiency, and the extract is not easy to mold. Ethanol is the most commonly used.
Lipophilic organic solvents: such as petroleum ether, chloroform, and ether, can extract fat-soluble components such as volatile oils, oils, and chlorophyll, but most of these solvents are flammable, toxic, and expensive.
Immersion method: After crushing the plant raw material, place it in a container, add an appropriate amount of solvent, and soak it at room temperature or low temperature for a certain period of time. This method is suitable for extracting heat-unstable components. The operation is simple, but the extraction efficiency is low and the solvent consumption is large.
Percolation method: put plant powder into the percolation cylinder, and continuously add solvent to make it permeate through the raw material layer to extract the effective ingredients. This method has a higher extraction efficiency than the immersion method, but the amount of solvent used is large and it takes a long time.
Decoction method: add water to the plant raw material and heat it and boil it for extraction. It is suitable for extracting water-soluble and heat-stable components, and the operation is simple, but it is not suitable for plants containing volatile and heat-destructible components, and the extract contains more impurities.
Reflux extraction method: use organic solvents to heat the extraction, and use the solvent repeatedly through the reflux device. The extraction efficiency is high, but it is not suitable for components that are easily destroyed by heat, and the solvent consumption is large, and the operation is more complicated.
Continuous reflux extraction method: use Soxhlet extractor to recycle the solvent during the extraction process, which can improve the extraction efficiency and save solvent, but the extraction time is long and it has an impact on components that are easily decomposed by heat.
Precautions
The raw materials need to be properly crushed to increase the contact area with the solvent, but too fine will easily lead to increased dissolution of impurities.
Select appropriate solvents and extraction methods according to the target components and raw material characteristics.
Control the extraction temperature, time and solvent dosage to improve extraction efficiency and product quality.
Pay attention to safety during the extraction process. When using organic solvents, be sure to prevent fire, explosion and poisoning.
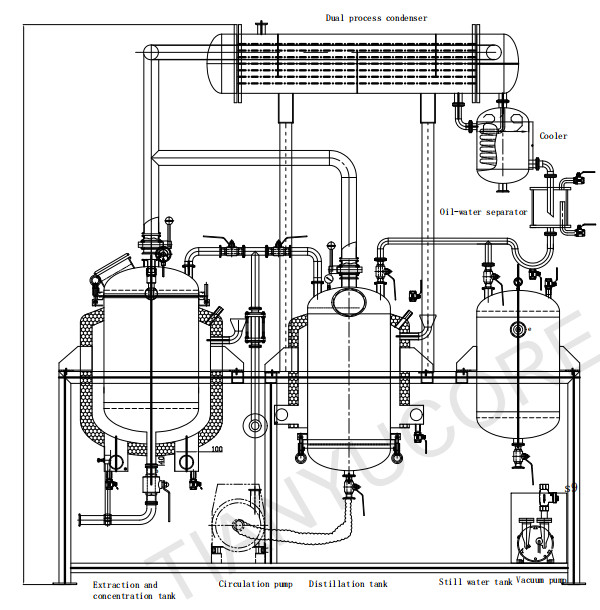
The following are the classifications of conventional raw materials suitable for solvent extraction and typical examples:
I. Applicable raw materials for hydrophilic solvents (such as water, ethanol, methanol)
Alkaloids
Raw materials: Coptis chinensis (berberine), Catharanthus roseus (vincristine)
Features: High polarity, requires extraction with polar solvents (such as ethanol).
Flavones/glycosides
Raw materials: Ginkgo biloba (flavonoid glycosides), Scutellaria baicalensis (baicalin)
Features: Easily soluble in water or ethanol, suitable for decoction or reflux extraction.
Polysaccharides
Raw materials: Lycium barbarum, Astragalus
Features: Requires hot water extraction, followed by separation by alcohol precipitation.
Amino Acids/Proteins
Raw Materials: Chrysanthemum, Soybean
Features: Extracted with cold or warm water to avoid high temperature destroying activity.
II. Applicable Raw Materials for Lipophilic Solvents (such as Petroleum Ether, Chloroform, Ether)
Volatile Oils
Raw Materials: Peppermint, Angelica, Cloves
Features: Cold Soaking with Low Boiling Point Solvents (such as Ether) or Steam Distillation Combined with Solvent Extraction.
Oils/Waxes
Raw Materials: Soybean, Cocoa Beans
Features: Soxhlet Extraction with Petroleum Ether, Used for Preparation of Oils or Removal of Impurities.
Pigments
Raw Materials: Turmeric (Curcumin), Chili (Capsanthin)
Features: Extracted with Chloroform or Acetone, Need to be Operated in Darkness.
III. Application of Special Raw Materials and Mixed Solvents
Raw Materials: Frankincense, Myrrh
Method: Dissolve the resin with a mixed solvent of ethanol + acetone.
Raw materials containing volatile oil and water-soluble components
Raw materials: dried orange peel (volatile oil + flavonoids)
Method: First, extract the volatile oil by steam distillation, and then use ethanol to extract the flavonoids in the residue.
IV. Selection suggestions
Select solvents according to target ingredients
Polar ingredients (glycosides, alkaloids) → ethanol/water
Non-polar ingredients (volatile oils, fats) → petroleum ether/ether
Pay attention to raw material characteristics
Heat-sensitive ingredients (volatile oils) → cold soaking or low-boiling point solvents
Contains a lot of starch/mucilage → first defatting or enzymatic pretreatment
Safety and cost considerations
Small-scale laboratory: ethanol is preferred (low toxicity, easy to recycle)
Industrial production: Consider solvent cost and safety (such as acetone instead of ether)
Typical application scenarios
Extraction of traditional Chinese medicine: baicalin (ethanol reflux), artemisinin (ether cold soaking)
Food additives: capsanthin (acetone extraction), tea polyphenols (water extraction + resin adsorption)
Cosmetics: Centella asiatica extract (ethanol percolation), vegetable oils and fats (Soxhlet extraction)
Black Pepper Oil Extraction
Molecular Distillation Equipment Application
Oleoresins Introduction
Contact: Project Manager
Phone: +86-18120438367
Tel: +86-18120438367
Email: info@tycoretech.com
Add: No. 1, Optics Valley Avenue, East Lake New Technology Development Zone, Wuhan, Hubei, China
We chat
