Before the advent of essential oils, people directly crushed fragrant plants for perfume making. With the accumulation of knowledge and the advancement of technology, people gradually invented methods to extract essential oils. The advent of essential oils has gradually ushered in a new chapter in perfume making.
Common extraction methods for essential oils
We have previously described the steam distillation method in detail. 95% of essential oils can be extracted by this method, but the following situations cannot be extracted by distillation:
· The aroma is not easy to volatilize
· Unstable under heat
· Easy to hydrolyze, etc.
At this time, other methods are needed, such as extraction, also known as leaching. Common ones include solvent extraction and supercritical CO extraction. This article will first explain the solvent extraction method in detail.
I. Overview
Volatile organic solvents are used to soak the fragrant parts of plants, so that these aromatic substances are transferred to the solvent, and then the solvent is evaporated to obtain an extract. The extract is then dissolved in ethanol, and solid impurities are filtered out after cooling. The ethanol is recovered by vacuum distillation to obtain the pure oil, which is the essential oil sold on the market.
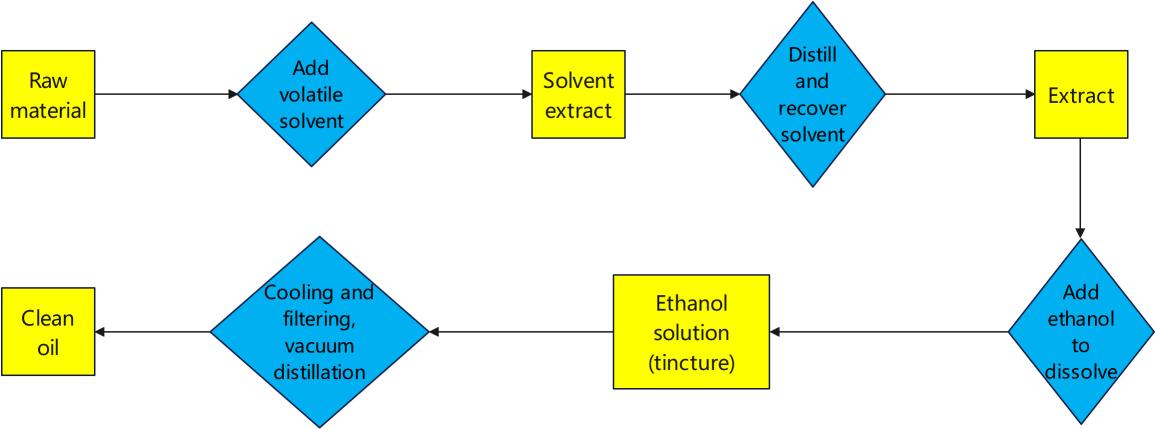
Flow chart of solvent extraction of essential oils
II. Detailed explanation of the principle
As you can see from the flow chart, the extraction method is more complicated than the distillation method. Therefore, the essential oil obtained by the extraction method is often sold at a higher price. The quality is slightly better in most cases, but not absolutely. Let's give a detailed explanation of each step below.
1. Fragrance raw materials
As mentioned above, the fragrance raw materials have the characteristics of not easy to volatilize, thermal instability, and easy hydrolysis. The raw materials commonly used for extracting essential oils by solvent extraction are: jasmine, osmanthus, dark red rose, rock rose, white orchid, tuberose (tuberose), tree moss, vanilla beans, musk, agarwood, etc.
The raw materials generally need to be pre-treated before soaking in order to make the aromatic substances more leached, such as cutting agarwood into pieces, fermenting vanilla beans, and drying raw materials with high water content in the shade first.
2. Add volatile solvents
The solvents should be colorless and odorless, insoluble in water, highly soluble in aromatic components but weakly soluble in impurities, with low boiling points and no residue after evaporation. Commonly used solvents include petroleum ether, ethanol, propane, butane, hexane, ether, ethyl acetate, etc., and petroleum ether (60~90℃) is the most commonly used solvent for extracting essential oils from fresh flowers.
(1) The ratio of solvent to aromatic raw materials should be just enough to cover the raw materials. The extraction time can be set to replace the raw materials every 24 hours until the individual feels that the solvent fragrance is suitable.
(2) The container should be sealed during extraction. If conditions permit, a Soxhlet extractor can be selected. During extraction, shaking, stirring, heating, etc. can be selected to shorten the extraction time of aromatic substances.
(3) The oil yield of the same raw material will vary with different solvents. For example, the oil yield of osmanthus is 7.35% when anhydrous ethanol is used as solvent, while the extraction rate is only 2.27% when ethyl acetate is used as solvent.
3. Solvent extract
The solvent extract at this time often contains more impurities. In addition to the aromatic components of the plant, it also contains plant wax, pigments, sugars, starch, fat, etc. Before distillation and recovery, it can be cooled and filtered and dried with anhydrous sodium sulfate to increase the purity of the essential oil.
If the solvent used is a medium with skin care effects such as vegetable oil, an appropriate amount of beeswax can be added to make it directly into a balm.

4. Distillation and recovery of solvent
Since the solvent used has the characteristics of low boiling point and high volatility, the temperature required for distillation and recovery will not be too high. This step can also be done by vacuum distillation to distill and recover the solvent at a lower temperature to ensure better retention of aromatic substances. When handling some flammable solvents, it is necessary to be particularly careful and stay away from fire.
5. Extract
Generally, we think that essential oils are the final clarified oily products, but in a broad sense, any extracted plant aroma components can be called essential oils. In order to distinguish them from the narrow sense of essential oils, the extracts here are generally called extracts, and the extracts can be used directly for perfumery.
6. Add ethanol to dissolve
Here, you should choose anhydrous ethanol or 95% ethanol. The less water content, the purer the essential oil will be. Ethanol can selectively dissolve the aromatic substances in the extract, and those impurities that are insoluble in ethanol can be filtered and separated.
7. Ethanol solution (tincture)
Here is a term - tincture. Tincture refers to the extract obtained by soaking aromatic raw materials with ethanol as a solvent. Tinctures are often used in the medical field. Here, if the volatile solvent in the first step is ethanol, then the "solvent extract" obtained is called tincture. Tincture can be used as the base alcohol for perfume.
8. Cooling filtration, vacuum distillation
Put the ethanol solution in the refrigerator to freeze the fat, wax, etc. in it to precipitate and crystallize, then filter it with special filter paper, coffee paper, or filter syringe, and then evaporate the ethanol through vacuum distillation to obtain the pure oil, which is the finished essential oil we see.
Black Pepper Oil Extraction
Molecular Distillation Equipment Application
Oleoresins Introduction
Contact: Project Manager
Phone: +86-18120438367
Tel: +86-18120438367
Email: info@tycoretech.com
Add: No. 1, Optics Valley Avenue, East Lake New Technology Development Zone, Wuhan, Hubei, China
We chat
